The gas exchange process in the lungs and blood involves the exchange of oxygen and carbon dioxide in the alveoli and tissues, and is influenced by the role of hemoglobin and the pH, temperature, and carbon dioxide concentration of the blood. This process plays an important role in regulating bodily functions by maintaining the proper concentration of oxygen and carbon dioxide.
Oxygen from the blood in the lungs is delivered to the heart and then to each tissue in the body to be used for energy production, while carbon dioxide, a waste product of metabolism, is delivered through the blood to the heart and then to the lungs to be expelled from the body. Gas exchange between blood and alveoli and blood and tissues occurs by diffusion due to partial pressure differences, and gases diffuse from higher to lower partial pressures. Among the blood vessels that carry blood, the blood vessels that flow from the heart to the lungs and tissues are called arteries, and the blood vessels that flow from the lungs and tissues to the heart are called veins. The blood that flows from the heart to each tissue after gas exchange occurs in the lungs is arterial blood, and the blood that flows from the tissues to the lungs after gas exchange occurs in the tissues is venous blood.
The partial pressure of oxygen in the alveoli is 100 to 110 mm Hg, and the partial pressure of oxygen in the venous blood in the capillaries surrounding them is 40 mm Hg, so the oxygen in the alveoli diffuses into the venous blood in the capillaries surrounding the alveoli. The oxygen-enriched blood then flows through the heart to each tissue in the body, and since the partial pressure of oxygen in the arterial blood flowing through the capillaries of each tissue is 100 mm Hg and the partial pressure of oxygen in the tissue is 40 mm Hg on average, the oxygen in the arterial blood diffuses into the tissue. The oxygenated blood flows through the heart to the lungs.
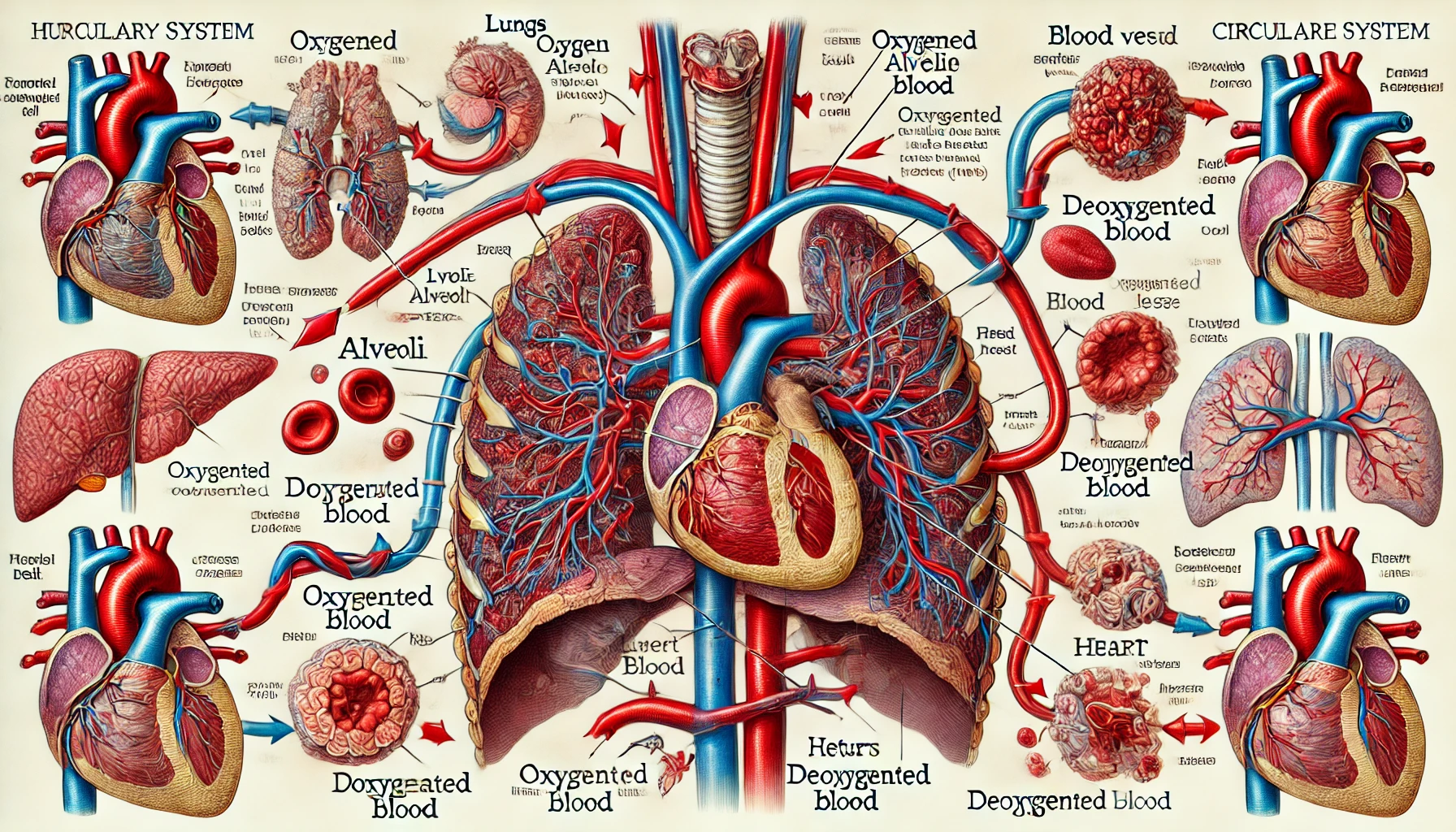
However, oxygen has a small solubility in water, so the amount transported in plasma is only about 1.5% of the oxygen transported from the lungs to the tissues, and about 98.5% is transported in the form of oxygenated hemoglobin by combining with hemoglobin in red blood cells. When hemoglobin combines with oxygen, it forms iron oxide, which is one of the reasons blood is red in color. During this process, hemoglobin maintains a structure that allows it to transport oxygen very efficiently. This process is essential for maintaining the body’s physiological balance, and the blood’s ability to carry oxygen is an important indicator of overall health.
The curve that shows the oxygen saturation of hemoglobin as a function of partial pressure of oxygen is called the oxygen dissociation curve, where the horizontal axis is the partial pressure of oxygen in the blood and the vertical axis is the oxygen saturation of hemoglobin. At any given partial pressure of oxygen, oxygen saturation, the degree to which hemoglobin is bound to oxygen, plus oxygen dissociation, the degree to which hemoglobin is separated from oxygen, equals 100%. The curve is a gentle S-shape, meaning that as the partial pressure of oxygen decreases, the amount of oxygen that dissociates from oxygenated hemoglobin is greater in the 0 to 40 mm Hg range than in the 40 to 100 mm Hg range. The oxygen affinity of hemoglobin refers to the tendency of hemoglobin to bind oxygen. In addition to partial pressure, factors that affect oxygen affinity include the pH (hydrogen ion concentration index) of the blood and temperature.
When metabolism in any tissue is active, the pH of the blood in the surrounding capillaries decreases due to an increase in carbon dioxide. When the pH of the blood is lowered, the oxygen affinity of hemoglobin becomes smaller, which promotes the dissociation of oxygen and the release of oxygen into the surrounding tissues. In other words, more oxygen is released from oxygenated hemoglobin at a lower pH when the partial pressure of oxygen is the same. In addition, in blood flowing through capillaries around tissues that have increased in temperature due to physical activity, such as exercise, oxygen dissociates more easily and more oxygen is released into those tissues than before exercise.
Changes in the pH of the blood are generally attributed to changes in the concentration of carbon dioxide in the blood. Carbon dioxide reacts with water in the blood to form carbonic acid, which in turn breaks down into hydrogen ions and bicarbonate ions. As the concentration of hydrogen ions increases, the pH of the blood decreases, which is an important factor in promoting the release of oxygen from tissues. This process also plays an important role in maintaining the acid-base balance in the body.
On the other hand, carbon dioxide, a waste product of metabolism in each tissue, is also diffused and transported in the blood. The partial pressure of carbon dioxide in tissues is 46 mm Hg on average, and the partial pressure of carbon dioxide in arterial blood is 40 mm Hg, so carbon dioxide in tissues diffuses into the blood flowing through the capillaries surrounding the tissues. About 7% of the carbon dioxide transported from tissues to the lungs is dissolved in plasma, and about 23% is transported as carbaminohemoglobin, which binds to hemoglobin in red blood cells. Unbound hemoglobin binds carbon dioxide more readily than oxygen-bound hemoglobin to form carbaminohemoglobin, making venous blood more useful than arterial blood for transporting carbon dioxide using hemoglobin.
And about 70% of carbon dioxide is transported as bicarbonate ions. In tissues, diffused carbon dioxide combines with water to form carbonic acid, mainly in red blood cells under the action of carbonic anhydrase, and carbonic acid is ionized into hydrogen ions and hydrogen carbonate ions. At this time, hydrogen ions are mainly combined with hemoglobin, and hydrogen carbonate ions are diffused into plasma and transported to the lungs. In the capillaries around the alveoli, the opposite reaction occurs: the hydrogen carbonate ions travel to the red blood cells, where they recombine with hydrogen ions to form carbonic acid, which is then converted into carbon dioxide and water by the action of carbonic anhydrase. The carbon dioxide produced in this process diffuses into the alveoli and is expelled from the body. Enzymes in red blood cells play an important role in this process, and if their function is impaired, the efficiency of gas exchange is reduced.
In addition, the concentration of carbon dioxide directly affects the rate and depth of breathing. High concentrations of carbon dioxide stimulate respiration, increasing carbon dioxide expulsion through the lungs, which is essential for maintaining acid-base balance in the body. Conversely, low concentrations of carbon dioxide cause respiratory depression, triggering a feedback mechanism that seeks to increase the concentration of carbon dioxide in the blood. This mechanism of respiratory regulation plays an important role in maintaining homeostasis.
Thus, the process of gas exchange in the human body is highly regulated, with many physiological factors interacting to maintain appropriate concentrations of oxygen and carbon dioxide. This is essential for maintaining overall body function and health, and even small imbalances can have serious repercussions.
 I’m a blog writer. I want to write articles that touch people’s hearts. I love Coca-Cola, coffee, reading and traveling. I hope you find happiness through my writing.
I’m a blog writer. I want to write articles that touch people’s hearts. I love Coca-Cola, coffee, reading and traveling. I hope you find happiness through my writing.