Most atomic nuclei in nature are stable, but when their atomic number exceeds 83, they become unstable, emit radiation, and transform into stable nuclei. Alpha, beta, and gamma rays are emitted in this process, which transforms the element into a more stable state. Radioactive decay has many applications, including dating the Earth and treating cancer.
Most atomic nuclei in nature are stable, but when their atomic number exceeds 83, the electrical repulsion between protons increases and they become unstable. These nuclei spontaneously emit radiation in an attempt to transform into another stable type of nucleus. The radiation emitted includes alpha, beta, and gamma rays, and the process of emitting these radiations and becoming a different type of stable nucleus is called radioactive decay.
Radioactive decay is an important phenomenon that occurs in nature, demonstrating the tendency of atomic nuclei to move from an unstable state to a stable state. This process explains the presence of radioactive isotopes in nature, which are also used to measure the age of the Earth. For example, radiocarbon dating using carbon-14 is very useful in archaeology. Radioactive decay processes also play an important role in astrophysics to understand the evolution of stars and the formation of elements produced after supernova explosions.
Alpha decay is the decay of radioactive elements that emit alpha rays, converting unstable nuclei with large mass numbers into stable nuclei with smaller mass numbers. The alpha rays emitted are from the nucleus of helium, which is composed of two protons and two neutrons. Therefore, when a radioactive element undergoes alpha decay, the number of protons and neutrons decreases by two each, which decreases the atomic number by two, and the mass number, which is the sum of protons and neutrons, decreases by four. If uranium-238 with 92 protons and 146 neutrons alpha decays, it becomes thorium with 90 protons and 144 neutrons, which can be represented by the nuclear reaction equation below.
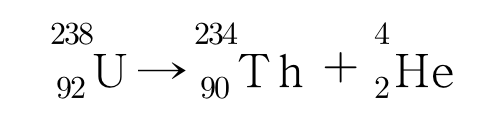
Beta decay, which emits beta rays, is the transmutation of an unstable nucleus with too many neutrons to protons into a more stable nucleus with fewer neutrons. In beta decay, the neutrons in the nucleus are turned into protons and electrons, and then the protons remain in the nucleus and only the electrons are released to the outside. In this case, one proton replaces the neutron that disappears, so the atomic number increases by one, but the mass remains unchanged. If cesium, which has 55 protons and 82 neutrons, undergoes beta decay, it becomes barium, which has 56 protons and 81 neutrons, with the following nuclear reaction formula
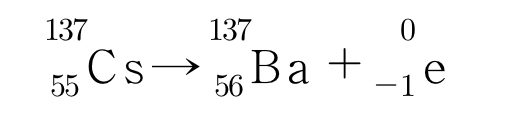
Gamma decay, which emits gamma rays, is the emission of energy in the form of electromagnetic waves to change the newly formed atomic nucleus from an unstable excited state to a stable state through alpha and beta decay. Therefore, gamma decay does not change the atomic number and mass number, and occurs in conjunction with alpha or beta decay, i.e., alpha and gamma rays are emitted together or beta and gamma rays are emitted together.
Naturally occurring radioactive elements stop decaying once they reach a stable state, such as lead. However, even stable atomic nuclei can be artificially made to emit radiation by bombarding them with high-speed particles from a particle accelerator to destabilize them. These artificially created radioactive elements are easy to produce and not expensive, so they have many applications in scientific research, industry, and medicine. For example, radioisotopes play an important role in cancer treatment. Certain radioisotopes can target and destroy cancer cells, which is an important part of radiation therapy.
As radiation travels through living organisms, it can cause ionizing radiation, which can lead to a number of biological changes, such as impaired cell division, mutations, and tissue destruction. Because of these properties, the handling of radioactive materials is highly regulated. Labs and hospitals that work with radioactive materials take various safety measures to minimize radiation exposure. Therefore, unlike the general public, people who work with radioactive elements must be careful not to expose themselves to more than a certain amount of radiation. Safe disposal of radioactive materials is also essential to protecting the environment and maintaining public health. Radioactive waste must be disposed of according to special procedures, for which a lot of research and technological development has been done.
As such, the decay of radioactive elements and the utilization of radiation play an important role in modern science and industry. The understanding and utilization of radiation will continue to evolve in the future, which will have a positive impact on many aspects of our lives.
 I’m a blog writer. I want to write articles that touch people’s hearts. I love Coca-Cola, coffee, reading and traveling. I hope you find happiness through my writing.
I’m a blog writer. I want to write articles that touch people’s hearts. I love Coca-Cola, coffee, reading and traveling. I hope you find happiness through my writing.