This article explains why chemical reactions are divided into voluntary and involuntary reactions, and how Gibbs free energy can be used to understand the spontaneity of a reaction.
When we look around us, we see many chemical reactions happening in real time in our bodies, whether it’s a series of white blood cells destroying pathogens in a television documentary or our mother roasting meat for dinner. In addition to these small reactions, there are also huge chemical reactions happening all around us, such as the way plants on Earth use the sun’s energy to make glucose, a form of energy that animals can use. How do these chemical reactions happen?
A chemical reaction is when one substance changes into another substance with different chemical properties, either by itself or by interacting with another substance. Chemical reactions can be viewed from different perspectives, such as from the energy perspective or from the rate perspective, but we will focus on the energy perspective. From an energy perspective, chemical reactions can be categorized as either spontaneous or involuntary. Spontaneous reactions are those that occur naturally without external stimuli, while involuntary reactions are those that require external stimuli.
In chemistry, we introduce the concept of Gibbs energy to quantify chemical reactions that may seem qualitative at first glance. The change in Gibbs energy tells us whether a chemical reaction is spontaneous or involuntary. Gibbs free energy is a thermodynamic function that is defined using the enthalpy, entropy, and temperature of a system, and is defined as follows
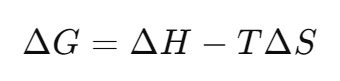
where G is the Gibbs free energy, H is the enthalpy, T is the absolute temperature, and S is the entropy. Where,

In light of the concept of Gibbs energy, we can see that a spontaneous reaction has a negative change in Gibbs energy. To understand why a reaction is spontaneous if the change in Gibbs energy is negative, it is convenient to think of the flow of water as a function of height. If the change in height of the water is negative, it means that the water will be lower later. Just as the movement of water from higher to lower is spontaneous, and a drop causes the water to move to a lower level, reactions are more spontaneous if they move from higher to lower energy, and all matter has a tendency to move to lower energy states, which are more stable. If you think of reactions that occur without any stimulus, you can think of oxidation reactions, such as rusting iron, or decomposition reactions, such as the acid hydrogen peroxide applied to a wound at the pharmacy, which breaks down into water and oxygen. As the name spontaneous suggests, it’s likely that most of the reactions we see around us are spontaneous. So how do these spontaneous reactions keep the Gibbs energy negative? Mathematically, the Gibbs energy is regulated by the relationship between enthalpy and entropy, so you can make the overall Gibbs energy change negative by making the enthalpy change negative (a reaction that releases heat), or you can make the entropy change positive (a reaction that increases disorder).
However, many of the reactions that take place in our bodies are actually involuntary, either reactions that decrease disorder, such as the synthesis of proteins from amino acids or the synthesis of DNA molecules, or reactions that absorb heat. These reactions are themselves involuntary and must be driven by an external source of energy, so you might wonder if there are more involuntary reactions around us than you think. This is because many of these involuntary reactions do not actually happen in isolation, but in combination with other large voluntary reactions, which means that if you add up the voluntary reactions that occur in combination with the involuntary reactions and view them as a larger reaction, the overall entropy change becomes positive, making the whole process voluntary. It’s like hanging weights of different weights on either side of a pulley. No matter how light the weight is, it will naturally fall down due to gravity, but if a heavier weight is suspended on the other side of the pulley, the lighter weight will rise up. From the perspective of the lightweight alone, this seems like an involuntary reaction, but when we look at the heavy and light weights together, it is natural for the heavier weight to go down and the lighter weight to go up. Thus, an involuntary reaction can be seen as part of a larger voluntary reaction.
In the modern world, many chemical reactions are controlled to make everything from trivial daily necessities to pharmaceuticals. It is important to find reactions that have higher yields or occur faster than conventional chemical reactions in order to obtain more or more expensive products. While this article does not address the rate of reaction or the equilibrium relationships of chemical reactions, it does introduce the most fundamental aspect of the process of finding such reactions: the spontaneity of the reaction, or “can this reaction happen?”.
 I’m a blog writer. I want to write articles that touch people’s hearts. I love Coca-Cola, coffee, reading and traveling. I hope you find happiness through my writing.
I’m a blog writer. I want to write articles that touch people’s hearts. I love Coca-Cola, coffee, reading and traveling. I hope you find happiness through my writing.